Introduction
Autologous stem cell transplantation (ASCT) has been used for treating multiple myeloma (MM) for over three decades and is generally reserved for patients younger than 65 years of age. The definition of transplant eligible is ill-defined and different centers have different policies to determine which patients are transplant eligible. Some centers have an age cut-off, others use clinical judgment, and some use various frailty scores (a scoring system based on comorbidities and physical and cognitive assessments) aiming to objectively assess transplant eligibility. There are limited data about outcomes in patients ≥ 75 years.
Aim
To report on outcomes of ASCT in a cohort of patients with MM aged 75 years or older.
Methods
Retrospective study of all consecutive MM patients aged ≥ 75 years that underwent ASCT at Mayo Clinic, Rochester, Minnesota. Stem cell transplantation at our center is routinely performed as an outpatient, with patients being hospitalized when deemed clinically necessary upon physician review.
Results
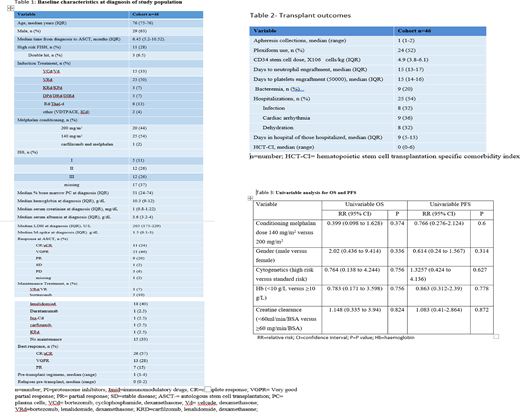
Between October 2005 and March 2020, 46 patients aged 75 years or older, received an ASCT at Mayo Clinic, Rochester. The median hematopoietic stem cell transplantation specific comorbidity index (HCT-CI) was 0 (range 0-6) with 8 patients having HCT-CI of 5 or 6. Median time from diagnosis to ASCT was 6.45 months (IQR 5.2-10.52) and 54% received reduced intensity conditioning with melphalan 140 mg/m2. All patients except one (that was treated with dexamethasone only) received induction with novel agents (listed in table 1) and 6 patients (13%) received doublet induction. All others received triplet induction. 46% of patients completed the ASCT without requiring hospitalization and 54% (n=25) of patients required hospitalization with a median duration of hospital admission of 9 days (IQR 5-13). Reasons for hospitalization included fever or infection (32%), cardiac arrhythmia (36%) and dehydration (32%). Overall response rate was 100% with a complete response seen in 57% of patients and 16 patients achieving MRD negative sCR. Median overall survival and progression free survival for the cohort were 82 months and 33 months, respectively. One patient died within 100 days of transplant representing a 2% 100-day mortality rate. Univariable cox regression model that evaluated the effect of gender, high risk cytogenetics, hemoglobin, renal function and melphalan dose did not detect any variable that was predictive of OS or PFS (Table 3).
Conclusions
ASCT is efficacious and can be safely delivered in the outpatient setting in carefully screened patients aged 75 or above. An arbitrary cutoff for age should not be used to exclude patients from ASCT, rather a careful assessment of "physiological age" including performance status and co-morbidities is required by an experienced treating team.
Kumar:Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Cellectar: Other; Carsgen: Other, Research Funding; Dr. Reddy's Laboratories: Honoraria; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Sanofi: Research Funding; Kite Pharma: Consultancy, Research Funding; Novartis: Research Funding; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Merck: Consultancy, Research Funding; MedImmune: Research Funding; BMS: Consultancy, Research Funding; Tenebio: Other, Research Funding; Karyopharm: Consultancy; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Genecentrix: Consultancy; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Adaptive Biotechnologies: Consultancy. Dispenzieri:Pfizer: Research Funding; Janssen: Research Funding; Alnylam: Research Funding; Intellia: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Dingli:Bristol Myers Squibb: Research Funding; Rigel: Consultancy; Janssen: Consultancy; Alexion: Consultancy; Karyopharm Therapeutics: Research Funding; Apellis: Consultancy; Sanofi-Genzyme: Consultancy; Millenium: Consultancy. Kapoor:Cellectar: Consultancy; Takeda: Honoraria, Research Funding; Celgene: Honoraria; Amgen: Research Funding; Sanofi: Consultancy, Research Funding; Janssen: Research Funding; GlaxoSmithKline: Research Funding. Gertz:Prothena: Other: personal fee; Medscape: Other: personal fee, Speakers Bureau; Appellis: Other: personal fee; Alnylam: Other: personal fee; Ionis/Akcea: Other: personal fee; Janssen: Other: personal fee; Research to Practice: Other; Sanofi: Other; Teva: Speakers Bureau; Johnson and Johnson: Speakers Bureau; DAVA oncology: Speakers Bureau; Proclara: Other; Springer Publishing: Patents & Royalties; Celgene: Other; Physicians Education Resource: Other: personal fee; Aurora Bio: Other; Amgen: Other: personal fee; Annexon: Other: personal fee; Spectrum: Other: personal fee, Research Funding; Abbvie: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal